Page 42 - 2022-bfw-morris-1e
P. 42
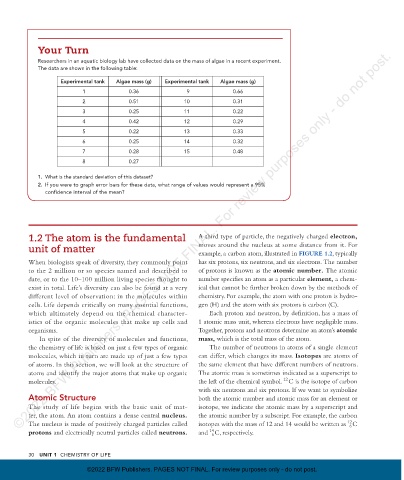
Your Turn
©2022 BFW Publishers. PAGES NOT FINAL. For review purposes only - do not post.
Researchers in an aquatic biology lab have collected data on the mass of algae in a recent experiment.
The data are shown in the following table:
Experimental tank Algae mass (g) Experimental tank Algae mass (g)
1 0.36 9 0.66
2 0.51 10 0.31
3 0.25 11 0.22
4 0.42 12 0.29
5 0.22 13 0.33
6 0.25 14 0.32
7 0.28 15 0.48
8 0.27
1. What is the standard deviation of this dataset?
2. If you were to graph error bars for these data, what range of values would represent a 95%
confidence interval of the mean?
1.2 The atom is the fundamental A third type of particle, the negatively charged electron,
unit of matter moves around the nucleus at some distance from it. For
example, a carbon atom, illustrated in FIGURE 1.2, typically
When biologists speak of diversity, they commonly point has six protons, six neutrons, and six electrons. The number
to the 2 million or so species named and described to of protons is known as the atomic number. The atomic
date, or to the 10–100 million living species thought to number specifies an atom as a particular element, a chem-
exist in total. Life’s diversity can also be found at a very ical that cannot be further broken down by the methods of
different level of observation: in the molecules within chemistry. For example, the atom with one proton is hydro-
cells. Life depends critically on many essential functions, gen (H) and the atom with six protons is carbon (C).
which ultimately depend on the chemical character- Each proton and neutron, by definition, has a mass of
istics of the organic molecules that make up cells and 1 atomic mass unit, whereas electrons have negligible mass.
organisms. Together, protons and neutrons determine an atom’s atomic
In spite of the diversity of molecules and functions, mass, which is the total mass of the atom.
the chemistry of life is based on just a few types of organic The number of neutrons in atoms of a single element
molecules, which in turn are made up of just a few types can differ, which changes its mass. Isotopes are atoms of
of atoms. In this section, we will look at the structure of the same element that have different numbers of neutrons.
atoms and identify the major atoms that make up organic The atomic mass is sometimes indicated as a superscript to
12
molecules. the left of the chemical symbol. C is the isotope of carbon
with six neutrons and six protons. If we want to symbolize
Atomic Structure both the atomic number and atomic mass for an element or
The study of life begins with the basic unit of mat- isotope, we indicate the atomic mass by a superscript and
ter, the atom. An atom contains a dense central nucleus. the atomic number by a subscript. For example, the carbon
12
The nucleus is made of positively charged particles called isotopes with the mass of 12 and 14 would be written as C
6
14
protons and electrically neutral particles called neutrons. and C, respectively.
6
30 UNIT 1 CHEMISTRY OF LIFE
©2022 BFW Publishers. PAGES NOT FINAL. For review purposes only - do not post.
04_morrisapbiology1e_11331_Unit1_Mod1_26-42_3pp.indd 30 10/04/21 9:09 AM